ABSTRACT
Flaviviridae-caused diseases are a critical, emerging public health problem worldwide. Flaviviridae infections usually cause severe, acute or chronic diseases, such as liver damage and liver cancer resulting from a hepatitis C virus (HCV) infection and high fever and shock caused by yellow fever. Many researchers worldwide are investigating the mechanisms by which Flaviviridae cause severe diseases.
Flaviviridae can interfere with the host’s innate immunity to achieve their purpose of proliferation. For instance, dengue virus (DENV) NS2A, NS2B3, NS4A, NS4B and NS5; HCV NS2, NS3, NS3/4A, NS4B and NS5A; and West Nile virus (WNV) NS1 and NS4B proteins are involved in immune evasion. This review discusses the interplay between viral non-structural Flaviviridae proteins and relevant host proteins, which leads to the suppression of the host’s innate antiviral immunity.
GENOME AND LIFE CYCLE OF FLAVIVIRIDAE
The Flaviviridae family includes a group of enveloped RNA viruses that contain a single-stranded positive-sense RNA genome approximately 9.4~13 kb in length. The Flaviviridae genome is composed of a polyprotein precursor flanked by a 5-terminal non-coding region (NCR) and a 3-terminal NCR. The polyprotein is processed by viral and host-cell proteases to produce approximately 10~12 mature proteins (including structural and non-structural proteins).
INNATE SENSING OF VIRUSES BY PATTERN RECOGNITION RECEPTORS
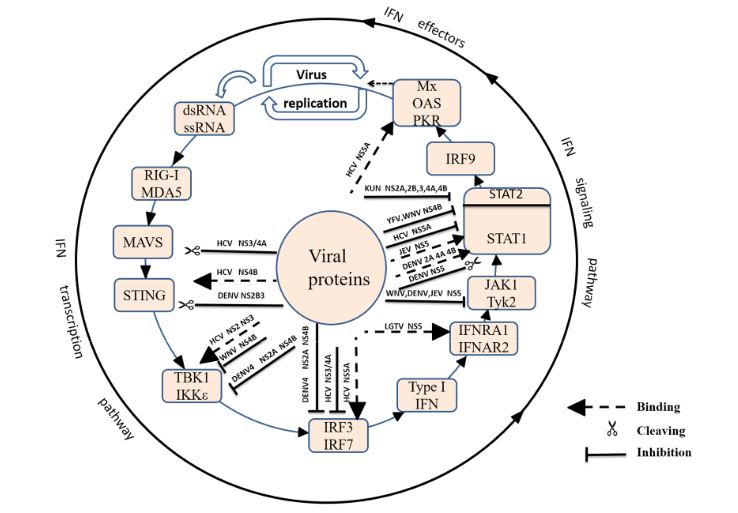
Figure 2. Suppression of type I IFN production by Flaviviridae viruses
Viruses are recognized by different types of pattern recognition receptors (PRRs) as pathogen-associated molecular patterns (PAMPs). PRRs, such as the Toll-Like receptors (TLRs) and retinoic acid-inducible gene I (RIG-I)-like receptors (RLRs), are activated by PAMPs, resulting in the activation of transcription factors and the production of cytokines, such as interleukin and interferon (Figure 2).
FLAVIVIRIDAE ANTAGONISM, IFN INDUCTION AND IFN-DEPENDENT SIGNALLING PATHWAYS
NS1 of Flaviviridae is an essential gene that generates a highly conserved ca. 48 kDa glycoprotein that is localized to the lumen of the ER by a signal sequence located at the C-terminus of the structural envelope protein E. NS1 is thought to be involved in the viral RNA replication process and the development of disease, because a deletion of the first YFV NS1 glycosylation site reduces the amount of viral replication and inhibits the secretion of NS1. The glycosylation site (Asn-207) and Cys residues of DENV NS1 is important in viral life cycle.
CONCLUSIONS
After long-term co-evolution of the virus and host, Flaviviridae has developed many strategies to evade the host’s innate immunity. First, the virus inhibits the induction of IFN. WNV NS1 and KUNV NS2A act on the TLR3 and RIG-I pathways, respectively, and DENV NS2B3 cleavage of STING results in the suppression of IFN β. Then, the virus inhibits the expression of antiviral molecules. DENV NS4B and TBEV NS5 inhibit the IFN-mediated phosphorylation of STAT. Finally, the virus abolishes the functional activity of antiviral molecules.
The interaction between HCV NS5 and PKR or 2′-5′ OAS inhibits the antiviral function of the ISGs. The Flaviviridae non-structural proteins play an important role in immune escape, but the signalling pathway and target sites of many non-structural or structural proteins remains unknown. Importantly, it is not known how they work together to interfere with the innate immune system. Future studies should not only deepen the understanding of immune evasion by Flaviviridae but also provide target sites for the development of new vaccines.
Source: Sichuan Agricultural University
Authors: Shun Chen | Zhen Wu | Mingshu Wang | Anchun Cheng