ABSTRACT
This manuscript details the results of the investigations into the effects of periodic low level temperature oscillations and pulsed variations in magnetic fields on the proliferation of multiple adherent human fibrosarcoma cells inside cell culture incubators. A custom built exposure system is used to characterize the dependence of cell proliferation changes on the frequency of the applied combined temperature and magnetic field variations. Small variations in the frequency of the oscillations in the vicinity of a “natural” frequency of the cells showed increased inhibition of cell proliferation.
Subsequently, using a previously developed shielding system for magnetic field shielding, the cells were subjected to pulsed variations in magnetic fields at constant temperature resulting in inhibition of proliferation to a smaller degree than in the earlier scenario. In order to ascertain the biological processes being affected by these temperature and magnetic field variations, levels of Nicotinamide Adenine Dinucleotide Phosphate (NADPH) were measured after the cells had been subjected to temperature and magnetic field variations and the results are reported here.
Although the effects of the variations are not well established, and the molecular mechanisms are currently being debated, this study attempts to show the link between the inhomogeneity in temperature and magnetic fields inside cell culture incubators and sources of variability in biological effects on cells such as proliferation, differentiation and death.
INTRACELLULAR BIOCHEMICAL OSCILLATIONS AND EXTERNALLY APPLIED PERIODIC STIMULI
Living organisms are constantly exposed to variations of temperature and electromagnetic fields as a result of natural and occupational causes. Even small variations in temperature and electromagnetic fields have the capacity to affect molecular structures and chemical reaction rates and facilitate synchronization of signals between cells. Research is ongoing to determine the effects of various low level electrical, magnetic and temperature fluctuations on biological cells.
EFFECT OF PHYSIOLOGICAL LEVEL TEMPERATURE OSCILLATIONS ON THE PROLIFERATION RATE OF HUMAN FIBROSARCOMA AND FIBROBLAST CELLS
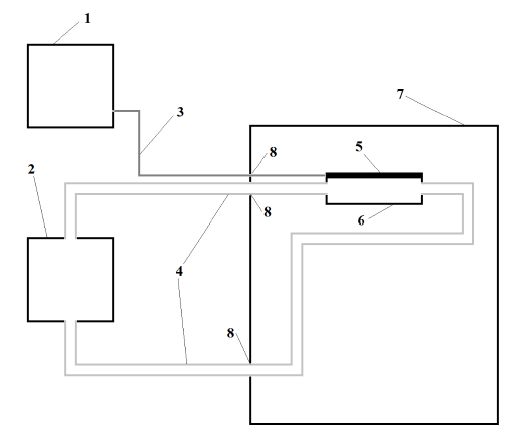
Figure 1: Schematic of thermoelectric platform system.
Figure 1: Schematic of thermoelectric platform system. (1) TPS Power supply; (2) 15 l Water bath and pump; (3) Electrical cable in twisted pair; (4) tubing for water circulation to- and from the thermoelectric platform; (5) thermoelectric culture platform; (6) thermoelectric platform polystyrene container; (7) host incubator; (8) modified incubator’s gasket.
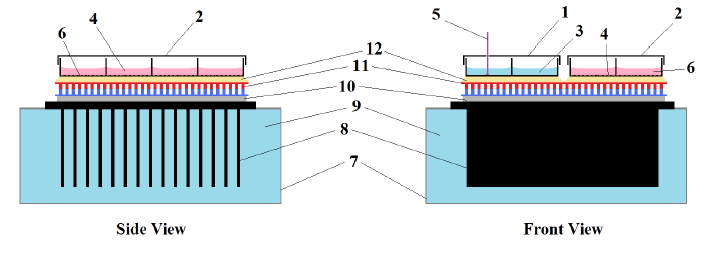
Figure 2: Thermoelectric cell culture platform.
Figure 2: Thermoelectric cell culture platform. (1) Temperature sensing 8-well slide probe; (2) Culture 8-well slide (exposed); (3) Water; (4) Adherent Cells; (5) Adherent cells; (6) cell culture medium; (7) 279cc polystyrene container with turbulent water flow; (8) Power dissipater fins; (10) Thin cyanoacrylate layer; (11) Thermoelectric module; (12) Thin immersion oil layer.
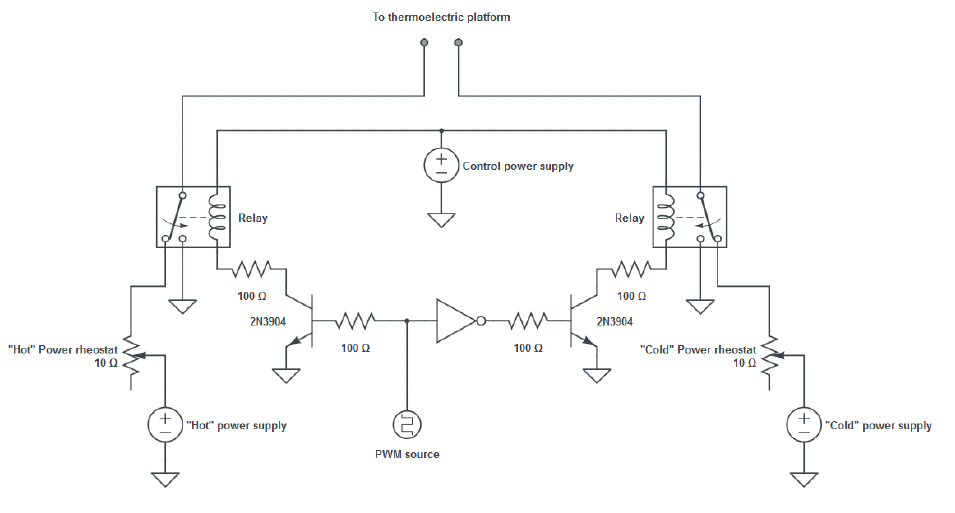
The power supply for this system was in the form of a biphasic square wave of variable period and duty cycle. As a result of the damping behavior of the system, which was similar to a low-pass filter, a delayed quasi-sinusoidal temperature signal was generated inside the cell culture wells. Two complementary power supplies in conjunction with a switching circuit in H-bridge configuration were used to generate this signal.
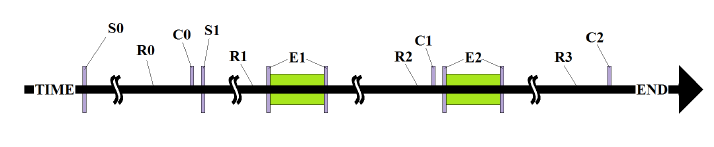
Figure 7: Typical exposure sequence. S0 = seeding (Passage 1) into T75 Flasks.
Figure 7: Typical exposure sequence. S0 = seeding (Passage 1) into T75 Flasks; S1 = seeding in 8- well slide (passages 4-12); R0, R1, R2 = “Rest periods” cultures were exposed to normal incubation conditions; E1, E2 = “Exposure periods” cultures were exposed to either oscillatory conditions (exposed) or normal incubation conditions on a non-working thermoelectric platform (control); C0, C1, C2 = “counts” cells numbers were assessed. Some experiments did not have a 2nd exposure (E2) and ended in C1 instead of C2.
EFFECT OF LOW LEVEL MAGNETIC FIELD PULSES ON THE PROLIFERATION RATE OF HUMAN FIBROSARCOMA AND FIBROBLAST CELLS
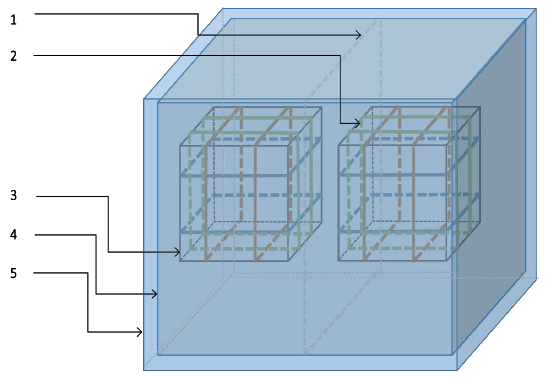
Figure 14: Assembled shielding and generation setup. (1) Central u-metal partition; (2) pulsed magnetic field generation coils; (3) geomagnetic field generation coils; (4) inner shield; (5) outer shield.
The coils were coated with weather resistant metallic coated tape and connected to the ground of the system in order to shield the cell culture area from electric fields generated by the presence of charges at different potentials as current flowed through the wires. One of these acrylic cylinders with the corresponding coil was used to maintain the internal volume at the earth’s magnetic field and served to house the control cell cultures, while the second cylinder and associated coil provided the pulsing magnetic fields as seen below.
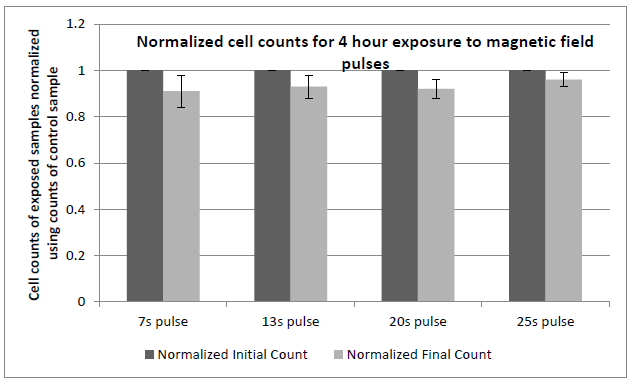
Figure 19: Comparison of cell proliferation rates of fibroblast cells exposed to pulsed magnetic field variations.
Figure 19: Comparison of cell proliferation rates of fibroblast cells exposed to pulsed magnetic field variations at the given frequencies when compared to control samples placed in geomagnetic field with no time varying fields. Cultures were exposed according to the sequence described earlier. 10,000 cells/ml were seeded in each well. Cell numbers in each well typically reached 50,000 cells/ml in normal incubation conditions (control) at the time here shown (50 hours after seeding). Experiments were repeated at least 3 times for the data shown. All exposures were done at 37+ 0.1°C. Error bars correspond to ± 1 standard deviation.
EFFECT OF LOW FREQUENCY TEMPERATURE OSCILLATIONS AND MAGNETIC FIELD PULSES ON NADPH LEVELS MEASURED IN HUMAN FIBROSARCOMA CELLS
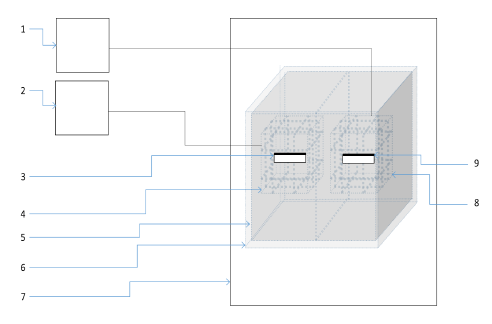
Figure 22: Schematic of pulsed magnetic field setup inside incubator.
Figure 22: Schematic of pulsed magnetic field setup inside incubator.(1) Power supply for geomagnetic field generation; (2) Power supply for pulsed magnetic field generation; (3) Cell culture platform for exposed cells; (4) Coils for pulsed magnetic field generation; (5)Inner shield; (6) Outer shield; (7) Cell culture incubator; (8) Coils for geomagnetic field generation; (9) Cell culture platform for control cells.
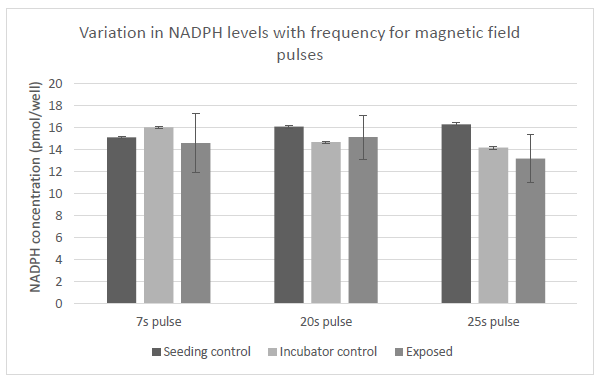
Figure 25: Variation in levels of NADPH in cells with exposure to magnetic field pulses. Control samples we placed in two different locations as denoted. Exposed samples were subjected to three different frequencies of magnetic pulses as labeled.
The above figure shows the variation in the levels of NADPH in cells that were subjected to pulsed magnetic field variations at the same frequencies as the previous set, i.e., 0.143Hz, 0.050Hz and 0.040Hz ( corresponding to time periods of 7, 20 and 25 seconds) according to the exposure sequence in Figure 7. Variation of the frequency at which the magnetic pulses were applied did not seem to result in statistically significant changes in the NADPH levels in the cells.
CONCLUSIONS AND FUTURE WORK
As part of this study we have constructed an experimental setup that can be used for the administration of different levels of temperature oscillations to multiple adherent cells being cultured simultaneously inside a cell culture incubator. Using this experimental setup, we have identified optimal parameters for exposure time, amplitude and seeding density for providing low frequency temperature oscillations to cells within the physiological range of temperatures. Our results show that that the proliferation of HT1080 human fibrosarcoma cells are inhibited by the action of these temperature oscillations.
We also assembled an experimental setup for administration of magnetic field pulses to multiple adherent cells being cultured simultaneously inside a cell culture incubator, using shielding and field generation components that were previously developed. Using this setup, we have identified optimal parameters for frequency of magnetic field pulses to inhibit cell proliferation of HT1080 cancer cells. We have also measured changes in NADPH levels as a result of the above experiments. However, before establishing a direct link between the applied stimuli and the intracellular processes, further real-time experiments measuring levels of NADPH, ROS and NO need to be undertaken.
Utilizing the experimental apparatus designed by us for the exposure of cells to temperature oscillations or magnetic field pulses, the effects of these stimuli on other kinds of cells can be studied. Construction of a modified setup using infrared heating of cells to provide temperature changes without the associated magnetic field perturbations. Finally, miniaturization of the setup that we have designed allowing for easier integration into existing cell culture incubators should be targeted.
Source: University of Colorado
Authors: Aditya Kausik